E-MOTIVE Trial
Early detection of postpartum haemorrhage and treatment using the World Health Organization MOTIVE ‘first response’ bundle: a cluster randomised trial with health economic analysis and mixed-methods evaluation.
The E-MOTIVE trial Nigerian arm was coordinated and conducted by the Africa Centre of Excellence for Population Health and Policy, Bayero University Kano. The trial was a RANDOMIZED TRIAL OF EARLY DETECTION AND TREATMENT OF POSTPARTUM HEMORRHAGE and demonstrated the concept of TRANSLATING RESEARCH FINDINGS TO POLICY IMPLEMENTATION which is one of the main aim of ACEPHAP.
Every six minutes a mother dies from PPH in low-resource countries, in the prime of her life and often leaving behind a young family. In many settings, when a mother dies in childbirth, her infant has less than a 20% chance of surviving past the first month. PPH, defined as a blood loss of more than 500 ml, is the leading cause of maternal death worldwide, accounting for 27% of maternal deaths. The WHO published “Recommendations for the Prevention and Treatment of Postpartum Hemorrhage” in 2012 to provide evidence-informed recommendations for managing PPH.3 However, adherence to these recommendations is currently limited by a number of challenges.
Challenges and proposed solutions:
1. PPH is often not detected early; thus life-saving treatment is not promptly initiated > Solution: Early detection and treatment of PPH
2. Delayed or inconsistent use of interventions for PPH management > Solution: the bundle
3. Despite guideline dissemination, many care providers do not provide effective care > Solution: Implementation strategy targeting Capabilities, Opportunities and Motivations for Behavior change (COM-B)
4. Limited scale-up and coverage Solution: Engagement of strong implementation partners: WHO/HRP, Jhpiego, UCL Centre for Behavior Change, and Concept Foundation
We evaluated the implementation of early detection and the use of the World Health Organisation (WHO) MOTIVE ‘first response’ treatment bundle for postpartum haemorrhage (PPH) on clinical, implementation and resource use outcomes. We evaluated the implementation through mixed-methods and carried out a health economic evaluation from the public healthcare system perspective.
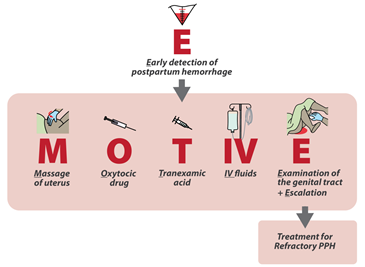
We conducted an international, cluster-randomized trial to assess a multicomponent clinical intervention for postpartum hemorrhage in patients having vaginal delivery. The intervention included a calibrated blood-collection drape for early detection of postpartum hemorrhage and a bundle of first-response treatments (uterine massage, oxytocic drugs, tranexamic acid, intravenous fluids, examination, and escalation), supported by an implementation strategy (intervention group). Hospitals in the control group provided usual care. The primary outcome was a composite of severe postpartum hemorrhage (blood loss, ≥1000 ml), laparotomy for bleeding, or maternal death from bleeding. Key secondary implementation outcomes were the detection of postpartum hemorrhage and adherence to the treatment bundle.
Multi-country, parallel cluster randomised trial with a baseline control phase, along with mixed-methods and health economic evaluations.
Secondary level health facilities in Kenya, Tanzania, Nigeria, South Africa, and Pakistan.
Population: Cluster: Health facility is the randomisation unit. Health facilities are eligible for inclusion if they have 1000 to 5000 births a year and provide comprehensive obstetric care with ability to perform surgery for PPH. Research participants: All healthcare providers attending vaginal births in the study facilities.
Intervention: The E-MOTIVE intervention consists of three elements: 1) a strategy for early detection of PPH, which allows triggering of the ‘first response’ treatment bundle; 2) a ‘first response’ bundle called “MOTIVE”, based on the WHO guideline recommendations and consisting of uterine Massage, Oxytocic drugs, Tranexamic acid, IV fluids and Examination & Escalation; and 3) an implementation strategy, focusing on simulation-based training with peer-assisted learning, local E-MOTIVE champions, feedback of actionable data to providers, calibrated drape with action line, and MOTIVE emergency trolley and/or carry case.
Control: Usual care with dissemination of the current guidelines.
A total of 80 secondary-level hospitals across Kenya, Nigeria, South Africa, and Tanzania, in which 210,132 patients underwent vaginal delivery, were randomly assigned to the intervention group or the usual-care group. Among hospitals and patients with data, a primary-outcome event occurred in 1.6% of the patients in the intervention group, as compared with 4.3% of those in the usual-care group (risk ratio, 0.40; 95% confidence interval [CI], 0.32 to 0.50; P<0.001). Postpartum hemorrhage was detected in 93.1% of the patients in the intervention group and in 51.1% of those in the usual-care group (rate ratio, 1.58; 95% CI, 1.41 to 1.76), and the treatment bundle was used in 91.2% and 19.4%, respectively (rate ratio, 4.94; 95% CI, 3.88 to 6.28).
1. WHO updating its guidelines
Following the outcome of the E-MOTIVE trial, and presentation of the results at various conferences, including the IMNCH, UNGA, the WHO have now developed 2 new recommendations on management of PPH incorporating the E-MOTIVE findings. This is going to be used throughout the world as the standard approach to PPH management
2. Nigeria has appointed two consultants to develop a new PPH guideline
Using the updated WHO guideline, FMOH Nigeria is developing its first ever National guideline for PPH care
3. Federal Ministry of health has approved the roll out of PPH detection and management using the E-MOTIVE bundle.
This will be done leveraging on the existing E-MOTIVE sites across the 6 geopolitical zones of Nigeria, and collaborating with partner organization also scaling up in their sites.
4. Local drape production
Calibrated blood collection drapes are now produced locally. This will enable the roll out process to be sustainable and make implementation cheaper and sustainable.
5. Partners keen to support the roll out in Nigeria
A number of developmental partners and NGOs operating in the health sector have indicated interest in supporting the roll out of the E-MOTIVE in their target facilities, states and regions. These include but not limited to: Bill and Melinda Gates foundation, T.A Connect, SOGON,
Early detection of postpartum hemorrhage and use of bundled treatment led to a lower risk of the primary outcome, a composite of severe postpartum hemorrhage, laparotomy for bleeding, or death from bleeding, than usual care among patients having vaginal delivery. (Funded by the Bill and Melinda Gates Foundation; E-MOTIVE ClinicalTrials.gov number, NCT04341662.)